Drosophila Connectomics: A Major Breakthrough in Neuroscience
A summary of the paper:
Dorkenwald, S., Matsliah, A., Sterling, A.R. et al. Neuronal wiring diagram of an adult brain. Nature 634, 124–138 (2024). https://doi.org/10.1038/s41586-024-07558-y
2025-07-15
Introduction
The year 2024 marks a milestone in neuroscience with the publication of the complete neuronal wiring diagram of an adult female Drosophila melanogaster brain by Dorkenwald and colleagues. This groundbreaking research encompasses 139,255 neurons and 54.5 million chemical synapses, representing the first connectome resource covering the entire brain. This achievement not only provides a valuable data foundation for neuroscience research but also opens new pathways for understanding how the brain works.
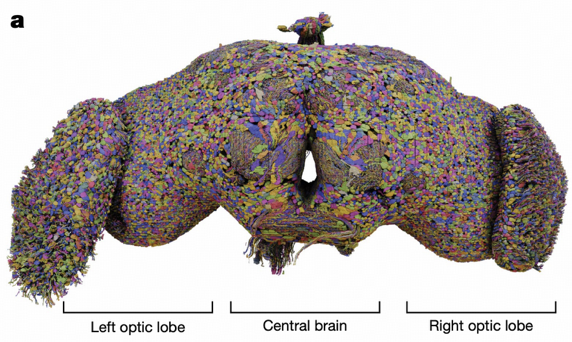
Connectomics: Mapping the Brain's "Circuit Diagram"
Connectomics is a science dedicated to mapping and studying neural connectomes, attempting to depict the complete wiring diagram of an organism's nervous system. It's like drawing a detailed "circuit diagram" for the brain, allowing us to understand how information flows and transmits within the nervous system.
The development of connectomics can be traced back to 1986, when scientists manually annotated electron microscope images to complete the connectome atlas of the nematode C. elegans's entire nervous system, achieving complete neural circuit reconstruction. This pioneering work laid the foundation for subsequent research.
In 2011, the construction of the rabbit retinal connectome marked the field's expansion toward more complex nervous systems. By the 2020s, the application of automated electron microscopy technology made large-scale connectome research possible, with scientists successfully reconstructing connectomes of Drosophila motor circuits, mouse visual cortex, and human brain cortex fragments.
The Significance of Drosophila Whole-Brain Connectome
Technological Breakthrough
The publication of the Drosophila whole-brain connectome represents a milestone in micro-connectomics, achieving complete insect brain reconstruction. This achievement was made possible by technological advances: from early static electron microscopy manual reconstruction to current automated image processing and large-scale data management.
In 2024, the proposal of LICONN technology provided new solutions for neural connection imaging. This technology combines hydrogel expansion and optical microscopy, opening new pathways for low-cost, high-efficiency, multi-channel neural connection imaging.
Research Value
Choosing Drosophila as a research subject has important advantages: the Drosophila melanogaster brain, while relatively small, is functionally complete, supporting complex behaviors such as vision, learning, and social interaction. Its brain contains approximately 10⁵ neurons, a scale that is complex enough to support advanced cognitive functions while being relatively simple for comprehensive study.
More importantly, parts of the Drosophila brain have been reconstructed through electron microscope images, with resolution sufficient to show neuronal branches and synapses. The resulting neural circuit connection atlases show similarities with mammalian brain circuits, providing important references for understanding more complex brains.
The Construction Process of Whole-Brain Connectome
Data Collection and Processing
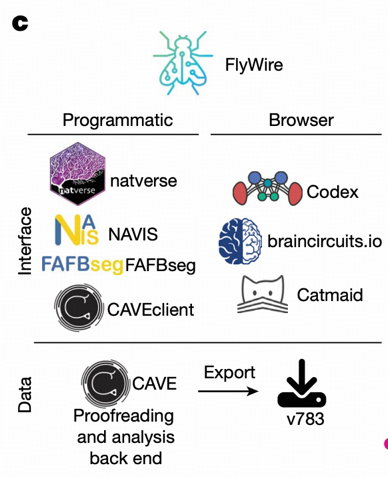
FlyWire community members shared 133,700 annotations of 114,209 neurons, demonstrating the important role of distributed collaboration in scientific research. Through the Codex platform (https://codex.flywire.ai), global researchers can collaborate in real-time for proofreading, ensuring data accuracy through this "crowdsourcing + expert review" quality control mechanism.
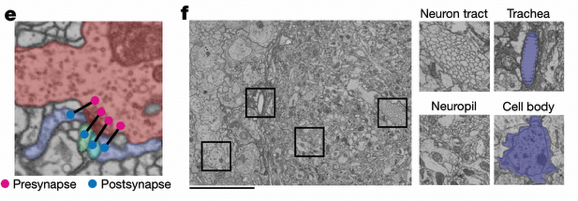

Neuronal Classification System
Based on three "flow" categories (afferent, intrinsic, and efferent), researchers classified neurons into nine superclasses. This classification method not only reflects the anatomical characteristics of neurons but, more importantly, reveals their functional roles in information flow.
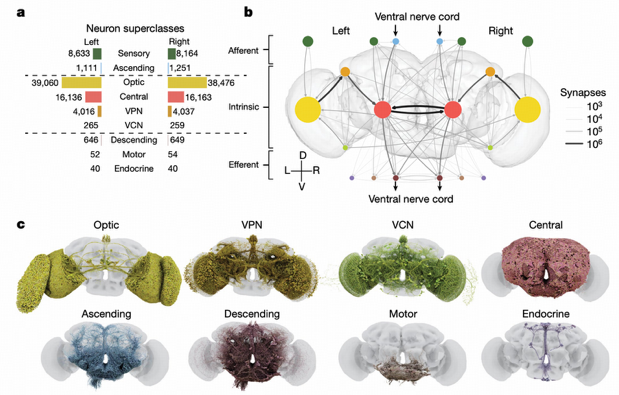
Afferent neurons (13.9%) include sensory neurons (visual, olfactory, gustatory, etc.) and ascending neurons. Among them, visual afferents are the most numerous, with compound eyes containing 11,118 neurons and ocelli containing 273 neurons. These neurons transmit information from the external world to the brain.
Intrinsic neurons (85%) have all synapses located within the brain. The high proportion means the brain primarily communicates with itself, and only secondarily with the external world. This ratio is much higher than in larval Drosophila (25-33%) and C. elegans (8-15%), reflecting the complexity and introspective nature of adult Drosophila brains.
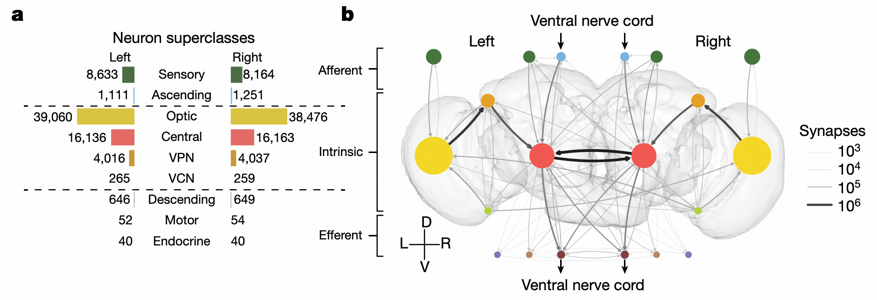
Efferent neurons (1.1%) include descending neurons, motor neurons, and endocrine neurons. Descending neurons mostly connect with the ventral nerve cord, driving motor behaviors, representing the direct pathway through which the brain affects the external world.
Analysis of Synaptic Connection Characteristics
Connection Density and Strength
The synaptic density in the Drosophila brain reaches 7.4/μm³, far higher than mammalian cortex (<1/μm³). This high-density connection provides the foundation for complex information processing.
Connection strength analysis shows that a single connection usually contains multiple synapses, and the number of synapses can be very large. Research found that 15,837 connections contain more than 100 synapses, with the strongest connection (VCN→mALC2) containing over 2,400 synapses. The median in-degree and out-degree of intrinsic neurons are 11 and 13 respectively, indicating that each neuron forms input connections with an average of 11 other neurons and output connections with 13 other neurons.
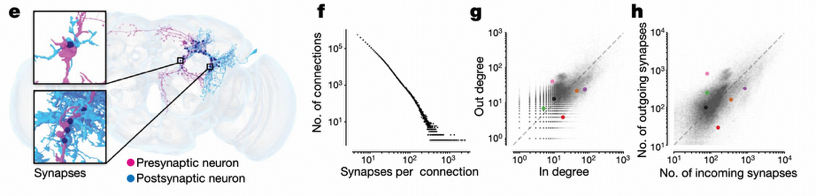
Neurotransmitter Distribution
Neurotransmitter analysis reveals the distribution patterns of excitatory and inhibitory neurons. Excitatory neurons mainly release acetylcholine, while inhibitory neurons mainly release GABA or glutamate. Notably, GABAergic neurons have higher connectivity (median in-degree 14, out-degree 16), indicating that inhibitory neurons play an important regulatory role in neural circuits.
Projectome: Information Flow Between Brain Regions
Projectome Matrix
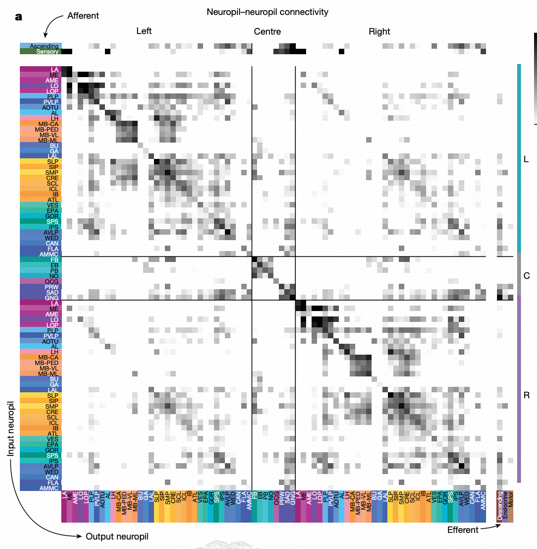
The projectome is a neuropil-neuropil matrix calculated based on intrinsic neuron connection patterns. Using 78 neuropil regions in the brain as basic units, each region serves as a row and column in the matrix, with each value representing the connection strength between two neuropils. This representation method intuitively shows information flow patterns between brain regions.
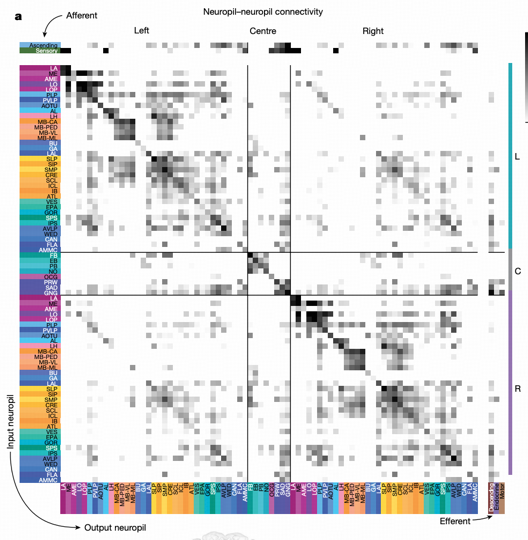
Key Findings
Projectome analysis reveals several important features: left-right hemisphere projection symmetry, with most projections being ipsilateral or between neuropils on the same side of the brain. The strongest cross-regional projections occur within the optic lobe (lobula→medulla) and central brain (mushroom body medial lobe→calyx).
Particularly important is the discovery of SEZ's (subesophageal zone) key role through projectome analysis. SEZ has connections with almost all brain regions, crucial for information flow in and out of the brain. Previous "hemibrain" studies did not cover this region, and this study's whole-brain connectome first completely presents SEZ's connection patterns.
Application of Information Flow Models
Information Propagation Mechanisms
While afferent and efferent neurons are small in number, they play key roles in connecting the brain with the external world. By examining these neurons' connections, researchers can predict intrinsic neuron functions and identify the shortest paths from afferent neurons to given intrinsic neurons.
This paper applies information flow models to Drosophila midbrain neuron connectomes: traversing the proportion of synaptic inputs from other neurons in neuron sets, propagating information probabilistically. The starting point of information flow is set as afferent neurons, including various sensory neurons.
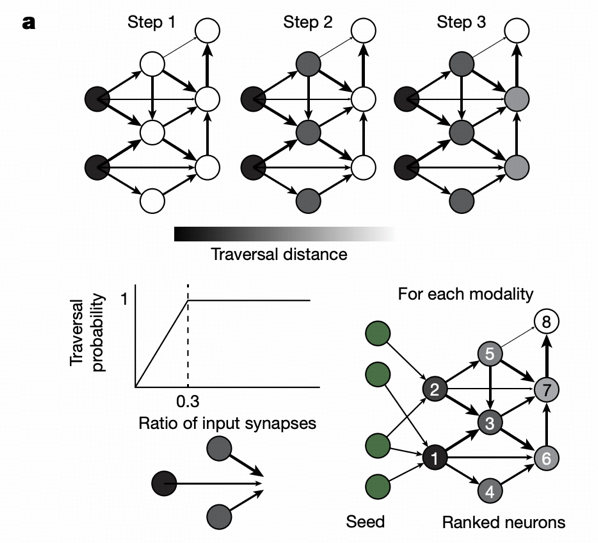
Small-World Properties
Information flow analysis reveals an important finding: almost all central brain neurons can be reached through any sensory modality, demonstrating small-world properties. Neurons predicted to express GABA and glutamate inhibitory neurons are significantly overrepresented among early-connected neurons, indicating the system prioritizes activating inhibitory neurons for preliminary regulation.
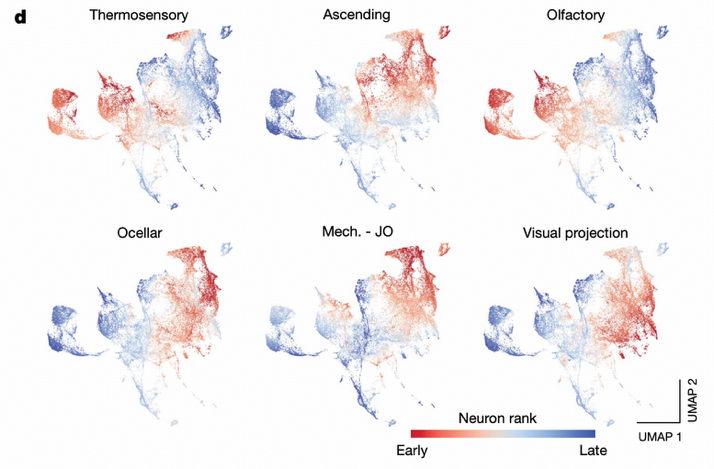
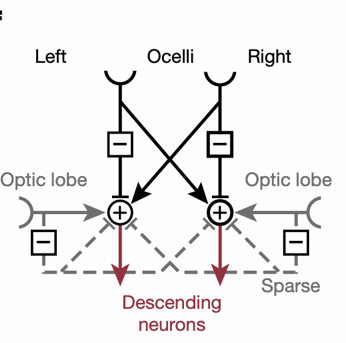
Case Study: Ocellar Circuit
Ocellar Function
Drosophila ocelli are located at the top of the head, sensing light changes to adjust flight posture and adapt to environmental light differences. Through fine reconstruction of the whole-brain connectome, researchers discovered neurons contained in the ocellar ganglion and constructed signal transmission pathways.
Neurotransmitter Differences
Intermediate neuron OCG01 receives direct input from photoreceptors, with half releasing glutamate (inhibitory), acting on ipsilateral downstream neurons; the other half releasing acetylcholine (excitatory), acting on contralateral downstream neurons. This "ipsilateral inhibition - contralateral excitation" mechanism provides the neural basis for rapid flight posture correction.
Flight Posture Correction Mechanism
When the Drosophila body deviates from the axis, such as tilting leftward, the left ocellus receives greater light intensity than the right. Through OCG01's "ipsilateral inhibition - contralateral excitation" mechanism, right descending neurons (such as DNp20) are activated, driving the head and body to roll rightward until both ocelli achieve balanced light intensity, achieving rapid flight posture correction.
Embodiment of Software Engineering Principles
Distributed Collaboration System
FlyWire achieved real-time collaborative proofreading by global researchers through the CAVE (Connectome Annotation Versioning Engine) system, embodying the "crowdsourcing + expert review" quality control mechanism common in AI data annotation. This distributed collaboration model not only improves work efficiency but also ensures data accuracy and consistency.
Algorithm Modularization and Toolchain Standardization
The neuronal automatic segmentation and synaptic detection Buhmann model in the paper integrates multiple independent algorithms, achieving process automation through tool libraries such as fafbseg and navis. This "algorithm modularization + toolchain standardization" approach embodies the idea of "API encapsulation" to improve system usability, providing a reusable technical framework for subsequent research.
Future Prospects: AI4Neuroscience
Algorithm Optimization
With the development of artificial intelligence technology, we can try to optimize image processing algorithms and probabilistic information flow models used in the paper. These optimizations will further improve the efficiency and accuracy of connectome research.
Automatic Recognition and Analysis
AI technology can help us achieve automatic recognition and principle analysis of neural circuits such as ocellar circuits. Through machine learning algorithms, we can discover new neural circuit patterns and functional mechanisms from massive connectome data.
The Challenge of Human Connectome
As H. Sebastian Seung said in "Connectome: How the Brain's Wiring Makes Us Who We Are": "I believe that before the end of the 21st century, we will have the opportunity to measure the entire human connectome. We will progress from nematodes to Drosophila, then conquer mice, followed by monkeys, and finally face the ultimate fortress—the human brain."
Conclusion
Drosophila whole-brain connectome research represents a major breakthrough in neuroscience. It not only first achieved complete mapping of Drosophila whole-brain neuronal connections, revealing global information flow mechanisms from sensory input to motor output, but more importantly, provided new methodological foundations for solving efficiency and precision challenges in whole-brain scale neuronal reconstruction.
Through the "automatic segmentation + community collaboration" model, researchers built the FlyWire ecosystem, laying the resource foundation for cross-species large-scale connectome research. While there are still some limitations in the research, such as electrical synapses not being identified and dendritic synaptic attachment rate only 44.7%, these point the direction for future technological development.
When future generations trace back our series of achievements, they will surely marvel at what an important scientific revolution this was. Drosophila connectome research not only allows us to understand brain working principles more deeply but also paves the way for humans to ultimately understand their own brains.
This article is based on my academic presentation, detailing the research background, technical methods, main findings, and future prospects of Drosophila connectomics. This research provides important tools for understanding the brain's "circuit diagram" and demonstrates the enormous potential of connectomics in neuroscience research.